Physical-Chemical analysis of fuels vapors
Interests/objectives
- Ecological assessment / PPA
- Physical-chemical properties of the compound in the gas phase
- RON an MON calculation of condensed gasolines
- Analysis and performances of Optimgaz system
|
 |
- Compounds found in the gaz phase or the automoble gazolineComposé(quantitative ans qualitative)
- Calculeted macroscopic properties
- Rate of abatement of hydrocarbons in gasoline
- Chromatograms of gasoline vapours
Interests/objectives
- Performance study of Optimgaz System in the condensation of VOC in gasoline (SP 98 and SP 95).
- To draw up an ecological assessment of the service station by calculating the rate of abatement of the VOC at the moment of the use of the Optimgaz system.
- To calculated the RON and the MON (octane number) of condensates given out of the tank.
- Integration of optimgaz system in the PPA ( Plan of Protection of the Atmosphere)
Recommended methods of analysis :
-
Gas Chromatography (qunatitative analysis of fuels),
-
Mass spectrometry qualitative analysis) et
-
DT/GC/MS
Physical-chemical types of selected analysis being methods known as sure and reliable, used largely in refineries and analysis laboratories.
Gas phase chromatography
Principle.
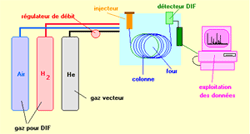
Gas chromatography (CPG) is, like all the techniques of chromatography, a technique which makes it possible to separate from the molecules of a mixture possibly very complex of very diverse nature and volatility.It applies mainly to the compounds gas or likely to be vaporized by heating without decomposition in the injector.The mixture to be analysed is vaporized in the entry of a column, with contains a activeted substance solid or liquid called stationary phase, then it is tranported through this one uing a carrying gas (or carrier gas).The column, placed in a thermostated furnace, is a tube of weak section rolled up on itself and containing the stationary phase. A great choice of detectors allows the analysis
selectiv and sometimes the identification of very complex mixtures.
The various molecules of the mixture will be separated and leave the column the ones after the thers aftre a certain lapse of time wich is a function of the affinity of the stationary phase withe these molecules.
Intrumentation.
| In the most traditional configuration, the chromatograph is equipped with a diving injector, a capillary tube and a ionization flame detector. The data are treated by an information processing system.
Fig.1 : Diagram of a GPC apparatus, provided with a ionization flame detector --> |
 |
Mass spectrometry
Principle| The mas spectrometry is a chemical technique of analysis making it possible to detect and identify molecules of interest by measurement of their monoisotopic mass. Moreover, the mass spectrometry allows to caracterize the chemical structure of the molecules by splitting up them. its principle lies in separation in gas phase of molecules charged (ions) according to their masse/charge report ratio (m/z). | 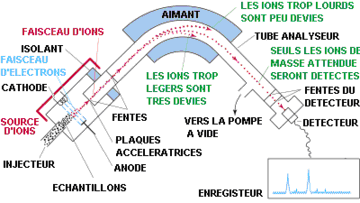 Fig. 2 - Diagram of the structure of a mass spectrometer: example of a mass spectrometer with a magnetic sector associated with a source of ionization of electronic impact. |
The system of thermic desorption /chromatography in phase gas/mass spectrometry.
The GPC/MS apparatus is used to determine the presence and the concentration of volatile organic compounds in gas samples.the majority of the VOV starting from C5 (pentane) until C16 (hexadecane) can be identified and quantified with a precision of the order of the ppbv. The samples are collected on adsorbent tubs on multiple levels. They are them desorbed on the thermal desorber connecte to the gas phase chromatograph/ mas spectrometer (GC/MS).The chemical compound are separated by high resolution capillary chromatography.The contents of detected and identified VOC are quantified by using toluene like external standard. The selected target compounds are identified and their contents quantified using indvidual standards.
 |
 |
| Fig. 3 - Adsorbent tubes | Fig. 4 - TG/GC/MS System |
Results / Conclusions
Compouds found in the gas phase of automobile gasoline (quantitative and qualitative)
Condensed products being in the gas phase of the automobile gsolines (SP98 and SP95) were analysed by high resolution gas chromatography and mass spectrometry.
Analysis method: NF M07-086 (ASTM 6733).
identification and quantification of various components by the automatic software of identification Carburane.
In the table below, you will find the name of the found chemical compound, its rough chemical formula, its developed semi formula (plane), its numbre CAS (Chemical Abstracts Service), the percent in weight in the gas phase and its topological formula.
| Chemical compound | % in the gasoline vapor | Topological formula |
| Isoparaffinics | ||
| isopentane C5H12 (CH3)2-CH-CH2-CH3 CAS: 78-78-4 |
35,7% | 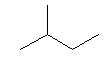 |
| 2,3-Dimethylbutane C6H14 (CH3)2CHCH(CH3)2 CAS: 96-14-0 |
6,2% |  |
| 2-Methylpentane C6H13 Isohexane /2-Methylpentane (CH3)2CH(CH2)2CH3 CAS: 107-83-5 |
5,1% |  |
| 2,2-Dimethylbutane C6H14 Neohexane; 2,2-Dimethylbutane (CH3)3CCH2CH3 CAS: 75-83-2 |
3,9% |  |
| 3-Methylpentane C6H14 (C2H5)2CHCH3 CAS: 96-14-0 |
2,6% | 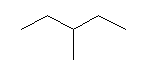 |
| 3-Methylhexane C7H16 2-Ethylpentane CAS: 589-34-4 |
0,7% | 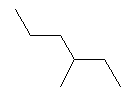 |
| 2-Methylhexane C7H16 CAS: 591-76-4 |
0,6% |  |
| 2,2,4-trimethylpentane C8H18 CAS: 540-84-1 |
0,6% |  |
| 1-Methylcyclopentane C6H12 CAS: 96-37-77 |
0,9% |  |
| isobutane C4H10 CAS: 75-28-5 |
0,9% |  |
| Oxygenateds | ||
| MTBE
C5H12O |
7,4% | 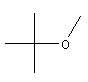 |
| ETBE C2H5-O-C4H9 (CH3)3C-O-CH2-CH3 Ethyl tertio butyle ether |
1,1% | |
| Paraffins | ||
| n-pentane C5H12 CAS: 109-66-0 |
4,5% | |
| n-butane C4H10 CAS: 106-97-8 |
3,7% | |
| n-hexane C6H14 CAS: 110-54-3 |
0,7% | |
| Aromatics | ||
| Toluene C7H8 CAS: 108-88-3 |
3,7% | 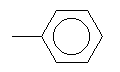 |
| Benzene C6H6 CAS: 71-43-2 |
0,7% | 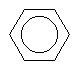 |
| Meta-xylene C8H10 CAS: 108-38-3 |
0,6% | 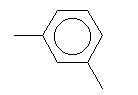 |
| Olefins | ||
| 2-methyl-2-butene C5H10 CAS: 513-35-9 |
3,3% |  |
| Trans-2-pentene C5H10 CAS: 646-04-8 |
2% | |
| 2-methyl-1-butene C5H10 CAS: 563-46-2 |
1,8% |  |
| Cis-2-pentene C5H10 CAS: 627-20-3 |
1,1% | |
| Isobutene C4H8 CAS: 115-11-7 |
0,6% |  |
| 1-pentene C5H10 CAS: 109-67-1 |
0,8% | |
| Trans-2-butene C4H8 CAS: 624-64-6 |
0,7% | |
| Cis-2-butene C4H8 CAS: 590-18-1 |
0,7% | 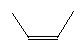 |
Macrocopic properties calculated
| Physical properties | Condensed |
| Density (15°C) | 0.6739 |
| Molar mass | 79.19 |
| Vapor tension (hPa) | 1005.54 |
| Calorific value (kJ/kg) | 46458.82 |
| Percentage of carbon | 83.16 |
| Percentage of hydrogen | 15.16 |
| Percentage of Oxygen | 1.68 |
| Paraffinic carbon | 82.67 |
| Naphtenic carbon | 2.74 |
| Olefinic carbon | 6.18 |
| Aromatic carbon | 8.40 |
| Vapor Pressure REID | 1064.79 |
| RON | 97.11 |
| MON | 84.91 |
These properties correspond to the properties of the gasoline condensed in petrol station, the vapors wich we condense can then be put in of tank after treatment.
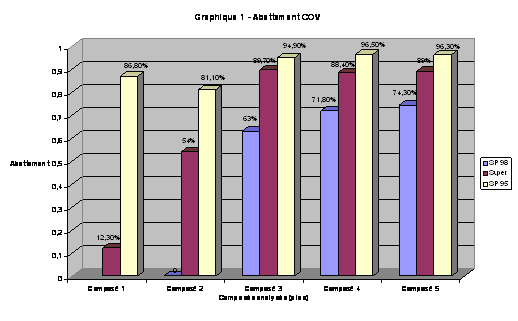
Percentge of abatement of hydrocarbons in the gasoline
VOC abatement of the most volatile compound present in automibile fuels by Optimgaz system. From the lightest compound (Compound 1) to the heaviest (Compound 5):
Chromatograms of gasoline vapors
Chromatographic signals before and after the cryogenic treatment Optimgaz ot SP 98 (gasoline). On the left, chromatographic analysis of gases before condensation and on the right, gas analysis after the passage and condensation by the cryogenic system:
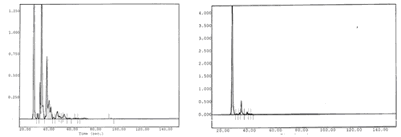
The large majority of VOC are condensed by the Optmgaz system: each chromatographic peak corresponds to a volatile organic material. The large peak wich remains (image of right-hand size) corresponds to the carrier gas.
The system of condensatio of fuel vapors Optimgaz reduces almost the totality of VOC at the moment of the discharges of tanker and at the moment of the filling of the automobile tanks.
These VOC These COV (BTEX --> benzene, toluene, ethylbenzene and xylenes [ ortho, meta and para ]) are the ozone precursors in the atmosphere - effect of greenhouse).
The use of the Optimgaz system contributes to reduce considerably the occupational diseases of the staff being in contact with the fuel vapors in petrol station (truck drivers, those wich are in kiosk of cashing...).
It is a system which finds its profitability at the end of a few months, because the fuel is recovered and given in the tank.



