Toxicology
Composition of the petroleum products
Fractions of distillation of oil
Three types of hydrocarbons considered in toxicology
 |
Chemical compounds of the vapour phase of the gasolines
and their toxicity
Composition of petrol compounds
The petroleum products are complex mixtures whose composition is variable according to the origin of the crude, the processes of refining and the processes of provisioning.
The refined products are:
-
Fuels: gasolines and high-grade petrols / gas oil / kerosene and gasolines aviation / fuels heavy marine fuels
-
The engine oils and hydraulics
-
Greases
-
Solvents
-
Natural gases and liquified
Fractions of distillation of oil
| Fractions of distillation of oil | Organic compounds | Utilisation |
| Natural gas | C1 – C2 | Fuel gaz |
| Liquid gas |
C3 – C4 |
Petrochemistry |
| Oil ether | C4 – C5 | Solvents |
|
Gasolines |
C5 – C10 |
Gasoline for aviation |
| Naphtha |
C6 –C10 |
Solvents – Fluids of cleaning |
| Kérosènes |
C5 – C16 |
Aviation fuels |
| Gas oil |
C9 – C16 |
Diesel |
| Paraffins | C20… |
Marine fuels |
| Tar | C20… | Asphalt, bitumens |
Three types of hydrocarbons considered in toxicology
Aliphatic hydrocarbons
Principal aliphatic HC:
-
Gazs: C1 à C4 : methane, ethane, butane
-
Volatile liquids : C5 à C8 : from pentane to octane
-
Not very volatile liquids : C9 à C16 : from nonane to hexadecane
-
Solids : + of C16 : tars, paraffins
Toxicity:
-
Methane, the ethane and propane are simple poison gases. The principal risk of methane is the explosion.
-
The vapors of higher hydrocarbons have an unquestionable toxicity acute and chronic.
Acute toxicity:
The symptoms of acute toxicity are summarized in the following table:
| Concentration | Answer |
|
550 ppm |
No effects |
| 900 ppm | Giddiness light, irritation of the eyes, nose, larynx |
| 1000 à 3000 ppm | Giddiness, more marked irritation of the mucous membranes, cephalgias, nauseas, anaesthesia |
| 10000 ppm | Giddinesses, irritation of the mucous membranes, coma in 4 to 10 m |
It was also described of pneumonias chemical (fever, dyspnea, severe hypoxemy, pulmonary infiltrate, turbid of the diffusion) for exposures to the hydrocarbon aerosols.
It should be known that the tanks of service station are more dangerous after having discharged their fuel than when they are full, because the vapors and the gases which are released in the cisterns can produce flamers mixtures, explosives and poison gases.
Chronic toxicity :
- Action on the central nervous system :
An exposure prolonged to the aliphatic hydrocarbon vapors, but also of hydrocarbons alicyclic and aromatic can support the development of behavioral deteriorations which could evolve/move in three phases:-1st phase: depressed syndrome ("organic emotional syndrome"): physical asthenia, psychic tiredness, depressive tendency, deceleration of the reaction times.
-2nd phase: syndrome dysphoric ("mild chronic toxic encephalopathy"): alternation of depression and irritability, complaints psychosomatic, deterioration of the psychomotor performances (short-term memory, attention, dexterity).
-3rd phase: syndrome psycho-organics ("severe chronic toxic encephalopathy") with attack of the mnemic and intellectual functions. The examinations tomodensitometric can show a diffuse cortical atrophy.
- Action on the skin:
The liquids alkanes (C5 to C16) are solvents of greases. The contact with the skin causes an irritation, sometimes a "dermo-épidermite". - Action on the lungs:
The chronic exposure to the hydrocarbon aerosols can cause a pneumopathy sometimes fibrosante.
Alicyclics hydrocarbons
One distinguishes:
- the cycloalcanes
- the cycloalcenes
- the cycloalcadienes
-
the terpenes (C10H16)
Their toxicity resembles that of corresponding alkanes
Aromatic hydrocarbons
These substances contain one or more benzene cores.Principal simple aromatic hydrocarbons:
All the fuels contain, in variable proportions, of aromatic hydrocarbons:
-
benzene up to 1% in the unleaded fuels )
-
alkylbenzenes (toluene, xylenes, polymethylbenzenes, ethylbenzenes, propylbenzenes, butylbenzenes
Toxicity of aromatic hydrocarbons :
-
Depression of the central nervous system of alcoholic intoxication type
-
Irritating action on the skin and the mucous membranes and irritation of the respiratory tracts.
The aspiration in the lungs of a few cubic centimeters of liquid hydrocarbons causes a severe hemorrhagic pulmonary oedema. -
Risk of toxic attack of the renal tubule (toluene) and of glomerular nephropathy.
- Ototoxicity: it was recently established for certain solvents, in particular toluene, xylenes and the styrene (Forge, 1999)
- Specific hematologic toxicity of benzene, cancerogenic some (class 1 of the CIRC): medullary aplasias and acute and chronic leukaemias.
BIBLIOGRAPHY
Forge A : Industrial chemicals are hazardous to hearing, The Lancet, 1999, 353, 1250
Hakkola M :Neuropsychological symptoms among tanker drivers with exposure to solvents. Occup Med (Oxf), 1994, 44 (5) ; 243-246
Lauwerys R :Toxicologie industrielle et intoxications professionnelles, 1992, 3ème édition, Masson éditeur, Paris.
Chemical compounds of the vapour phase of the automobile gasolines and their toxicology
Condensed products being in the gas phase of the automobile gasolines (SP98, SP95) were analyzed by gas chromatography high resolution and by mass spectrometry.
Method of analysis NF M07-086 (ASTM 6733).
Identification and quantification of the various components by the automatic software of identification Carburane.
In the table below, you will find the name of the found chemical compound, its rough chemical formula, its séveloppée semi formula, its number CAS (Chemical Abstracts Service), the percentage in weight in the gas phase, its topological formula and below each compound its toxicity.
| Chemical compound | % in the gasoline vapors | Topological formula |
| Isoparaffins | ||
| isopentane C5H12 (CH3)2-CH-CH2-CH3 CAS: 78-78-4 |
35,7% | 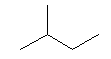 |
| Effect of the exposures of short duration: The substance is irritating for the eyes, the skin and the respiratory tracts. The ingestion of the liquid can involve an aspiration on the level of the lungs with a risk of pneumopathy. The substance can have effects on the central nervous system and the heart, involving a fonction insufficiency |
||
| 2,3-Dimethylbutane C6H14 br> (CH3)2CHCH(CH3)2 CAS: 96-14-0 |
6,2% | 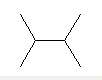 |
| Absorption This product is absorbed by the respiratory tracts and the digestive tracts. Acute effects Exposure to the vapors: possibility of irritation of the eyes and the throat and of depression of the central nervous system (headaches, nauseas, dizzy spells); aspiration in the lungs: chemical possibility of neumonite and pulmonary oedema. |
||
| 2-Methylpentane C6H13 Isohexane 2-Methylpentane (CH3)2CH(CH2)2CH3 CAS: 107-83-5 |
5,1% | 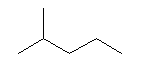 |
| Absorption This product is absorbed by the respiratory tracts and the digestive tracts. Acute effects Exposure to the vapors: possibility of irritation of the eyes and the throat and of depression of the central nervous system (headaches, nauseas, dizzy spells); aspiration in the lungs: chemical possibility of neumonite and pulmonary oedema. |
||
| 2,2-Dimethylbutane C6H14 Neohexane 2,2-Dimethylbutane (CH3)3CCH2CH3 CAS: 75-83-2 |
3,9% | 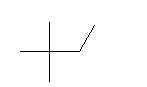 |
| 3-Methylpentane C6H14 (C2H5)2CHCH3 CAS: 96-14-0 |
2,6% | 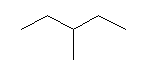 |
| Ways of exposure: The substance can be absorbed by the organization by inhalation of its vapors. Inhalation risk: A dangerous contamination of the air is quickly reached at the time of the evaporation of this substance at 20°C. |
||
| 3-Methylhexane C7H16 2-Ethylpentane CAS: 589-34-4 |
0,7% | 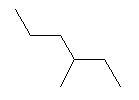 |
| 2-Methylhexane C7H16 CAS: 591-76-4 |
0,6% |  |
| 2,2,4-trimethylpentane C8H18 CAS: 540-84-1 |
0,6% | 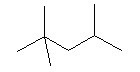 |
| Exposure of short duration: eyes, skin, respiratory tracts, kidneys, liver. Exposure of long duration: skin, kidneys, liver. Ways of exposure: inhalation, skin, eyes, ingestion. Symptoms: confusion, dizzy spells, cephalgias, nauseas, vomiting. |
||
| 1-Methylcyclopentane C6H12 CAS: 96-37-77 |
0,9% | 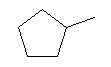 |
| isobutane C4H10 CAS: 75-28-5 |
0,9% | 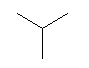 |
| Nausea, respiratory difficulty | ||
| Oxygenated compounds | ||
| MTBE C5H12O CH3-O-C(CH3)3 Methyl tert-butyl ether Propane, 2-methoxy-2-methyl CAS: 1634-04-4 |
7,4% | 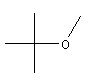 |
| The fugitive emissions coming from the gasoline refineries and the service station constitute the principal point sources of MTBE in the environment; the vehicles themselves emit sufficient MTBE to represent a significant source in the areas where circulation is dense.The surface water can be contaminated by gasoline discharges and the petrol engine boats, in particular those with engine at two times. Being given the volatility of the MTBE, the greatest part loses itself by evaporation before constituting a problem as regards drinking water. Discharges and the leakages of storage tanks can be at the origin of more serious problems in subsoil waters. The MTBE is not adsorbed much on the particles of the ground and is regarded as mobile (Canada Environment, 1993). It is also resistant to the chemical and microbial decomposition in water (ATSDR, 1996). This is why it is considered that the MTBE can represent a serious long-term threat for the drinking water provisioning if its use in the gasoline spreads. One has few scientific data on the effects on health of the MTBE present in drinking water. The studies carried out indicate that the MTBE can be cancerogenic in the animals, but they comprise all of the gaps which make them unusable to work out a recommendation on drinking water founded on criteria of a medical nature. | ||
| ETBE C2H5-O-C4H9 (CH3)3C-O-CH2-CH3 Ethyl tertio butyle ether |
1,1% | |
| Paraffins | ||
| n-pentane C5H12 CAS: 109-66-0 |
4,5% |  |
| n-butane C4H10 CAS: 106-97-8 |
3,7% |  |
| Ways of exposure: inhalation, skin, eyes. Symptoms: somnolence, gelures at the time of a contact with the liquid, narcosis, asphyxiates. Target bodies and ways of penetration: central nervous system. | ||
| n-hexane C6H14 CAS: 110-54-3 |
0,7% |  |
| Aromatics | ||
| Toluene C7H8 CAS: 108-88-3 |
3,7% | 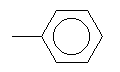 |
| Chronic toxicity A risk of tératogenèse is not to fear when the maximum concentration on the place of work is respected . Other toxicological information In the case of inhalation: Irritations of the respiratory tracts. In the case of contact with the skin: irritations. After prolonged action of the chemical: dermatite. Effect degreasing on the skin, possibly with secondary ignition. Danger of cutaneous resorption. in the event of contact with the eyes: irritation irritations of the mucous membranes. In the case of ingestion, faintness and vomiting. Danger of aspiration in the event of vomiting, resorption. Systemic effects: In the case of resorption of great quantities: disorders of the central nervous system, intoxication, spasms, unconsciousness, respiratory stop, cardiovascular insufficiency. |
||
| Benzene C6H6 CAS: 71-43-2 |
0,7% | 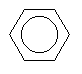 |
| Acute exposure The exposure to several hundreds of ppm acts on the central nervous system in particular involving states of somnolence, intoxication and headaches; weaker but prolonged exposures can deteriorate the memory and certain psychic capacities. Lastly, the benzene is responsible for irritating effects on the skin and the mucous membranes (eyepiece and respiratory in particular). Chronic exposure This substance is distinguished, for the mankind, by its great toxicity for the blood cells and the bodies which produce them (osseous marrow). This appears by a reduction of the red, white globules or plates. The importance of these demonstrations is a function of the benzene amounts to which the subject is exposed, of the simple anaemia to the attack of the three cellular lines (pancytopénie). This affection which constitutes the benzolism is currently completely exceptional in the countries developed because of the reduction of the levels of exposure. The affection which worries more, so much at the professional level than environmental, occurred of leukemias related to the exposure repeated to benzene concentrations of some ppm during several tens of years. Indeed, this one causes certain leukaemias myélo?des but other forms were highlighted in various studies. These attacks would more frequently occur after weak and continuous exposures rather than high and intermittent (peaks of pollution); they are often preceded by some by the blood anomalies described above. In addition, it was shown in the animal that the benzene can induce transmissible genetic deteriorations with the descent. e. |
||
| Meta-xylene C8H10 CAS: 108-38-3 |
0,6% | 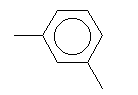 |
| Acute exposure Like all oil hydrocarbons liquidate distilling below 300°C, xylenes are irritating and depressors of the SNC. When they are introduced, they produce an acute abdominal syndrome and a pneumopathy of inhalation. Chronic exposure Xylenes do not involve in general effects specific distinguishing them from other oil hydrocarbons distilling to the lower part of 300°C. - xylenes involve dermites of chronic irritation (dry and squameuse skin). - Several studies brought back a particularly high frequency of néphropathies tubular and glomerular, as well as minor digestive disorders (anorexia, nauseas, diarrhoeas) at the employees exposed to xylenes. - In experiments, xylenes, with strong amounts, are foetotoxic and teratogenic in the rat. The current toxicological data do not make it possible to evaluate their cancerogenicity. They are not hémato-poisons. |
||
| Olefins | ||
| 2-methyl-2-butene C5H10 CAS: 513-35-9 |
3,3% | 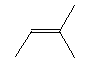 |
| Trans-2-pentene C5H10 CAS: 646-04-8 |
2% |  |
| 2-methyl-1-butene C5H10 CAS: 563-46-2 |
1,8% | 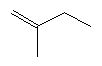 |
| Cis-2-pentene C5H10 CAS: 627-20-3 |
1,1% |  |
| Isobutene C4H8 CAS: 115-11-7 |
0,6% | 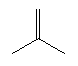 |
| Giddinesses. Somnolence. Weakening. Nauseas. Loss of conscience. Vomiting. Gelures at the time of the contact with the liquid. | ||
| 1-pentene C5H10 CAS: 109-67-1 |
0,8% |  |
| Trans-2-butene C4H8 CAS: 624-64-6 |
0,7% | 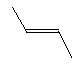 |
| Giddinesses. Loss of conscience. Gelures at the time of the contact with the liquid. | ||
| Cis-2-butene C4H8 CAS: 590-18-1 |
0,7% | 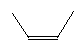 |
| Giddinesses. Loss of conscience. Gelures at the time of the contact with the liquid. | ||
References :
Cards of toxicology of INRS
International cards of chemical safety
http://training.itcilo.it/actrav_cdrom2/fr/osh/ic/nfrnsynb.htm International cards INRS CDC Centers for Desease Control and Prevention www.cdc.gov The whole of information and the toxicological data quoted above comes from various monographs published by organizations recognized for scientific quality their documents (ATSDR, OMS-IPCS, US EPA (IRIS)).



